Substituted tetrahydroisoquinoline


A substituted tetrahydroisoquinoline is a tetrahydroisoquinoline with one or more chemical substituents.[1][2] Many simple tetrahydroisoquinoline alkaloids related to mescaline are known and occur naturally in cactus species such as peyote (Lophophora williamsii) and Pachycereus pringlei among many others.[1][2][3][4] Simple tetrahydroisoquinolines may be thought of as cyclized phenethylamines.[1][2] As an example, anhalinine may be thought of as a cyclized analogue of mescaline.[1][2] The simple tetrahydroisoquinolines are analogous in concept to the β-carbolines and harmala alkaloids, which can be considered cyclized analogues of tryptamines.[5]
Some of the simple tetrahydroisoquinolines, for instance pellotine, are known to be pharmacologically active, although none are known to have hallucinogenic activity.[1][2][4] Known activities of simple tetrahydroisoquinolines include sedative and hypnotic effects, monoamine oxidase inhibition, and convulsant effects, among various others.[4][2] In the 2020s, various simple tetrahydroisoquinolines, like pellotine, were identified as serotonin 5-HT1D receptor ligands, serotonin 5-HT6 receptor partial agonists, and/or serotonin 5-HT7 receptor inverse agonists.[6][7] These actions, such as the serotonin 5-HT6 and/or 5-HT7 receptor interactions, may be involved in the sedative and hypnotic effects of some of these compounds.[6][7]
Synthetic tetrahydroisoquinoline analogues of phenethylamines, including AMPH-CR, METH-CR, PMMA-CR, DOM-CR, N-methyl-DOM-CR, DOB-CR, TDIQ (MDA-CR), and MDMTHIQ (MDMA-CR), have been developed and characterized.[8][9][10][11][12] In general, cyclization of stimulant, entactogen, and/or psychedelic phenethylamines into the corresponding tetrahydroisoquinolines results in abolition of the defining effects of these drugs as well as loss of their affinities for monoamine transporters and serotonin 5-HT2 receptors.[8][9][10][11][12] However, some of the tetrahydroisoquinoline forms, such as TDIQ, show selective affinity for α2-adrenergic receptors and associated effects.[12][10]
List of simple tetrahydroisoquinolines
[edit]Simple tetrahydroisoquinoline alkaloids
[edit]| Structure | Name | Chemical Name | PEA Counterpart[a] |
|---|---|---|---|
 | Tetrahydroisoquinoline (THIQ) | 1,2,3,4-Tetrahydroisoquinoline | β-Phenethylamine |
 | Longimammosine | 2-Methyl-6-hydroxy-THIQ | meta-Tyramine |
 | Longimammidine | 2-Methyl-8-hydroxy-THIQ | meta-Tyramine |
| Longimammatine | 6-Methoxy-THIQ | 3-Methoxyphenethylamine | |
| Weberidine | 7-Methoxy-THIQ | 4-Methoxyphenethylamine | |
 | Norsalsolinol | 6,7-Dihydroxy-THIQ | Dopamine |
 | Longimammamine | 2-Methyl-4,8-dihydroxy-THIQ | meta-Octopamine |
 | Salsolinol | 1-Methyl-6,7-dihydroxy-THIQ | Dopamine |
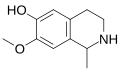 | Salsoline | 1-Methyl-6-hydroxy-7-methoxy-THIQ | 3-Hydroxy-4-methoxyphenethylamine |
 | Isosalsoline | 1-Methyl-6-methoxy-7-hydroxy-THIQ | 3-Methoxytyramine |
 | N-Methylisosalsoline | 1,2-Dimethyl-6-methoxy-7-hydroxy-THIQ | 3-Methoxytyramine |
 | Arizonine | 1-Methyl-7-methoxy-8-hydroxy-THIQ | 3-Hydroxy-4-methoxyphenethylamine |
| Corypalline | 2-Methyl-6-methoxy-7-hydroxy-THIQ | 3-Methoxytyramine | |
| Isocorypalline | 2-Methyl-6-hydroxy-7-methoxy-THIQ | 3-Hydroxy-4-methoxyphenethylamine | |
 | Uberine | 2-Methyl-5-methoxy-7-hydroxy-THIQ | 2-Methoxy-4-hydroxyphenethylamine |
 | Hedycarine | 1-(Hydroxymethyl)-2-methyl-6-methoxy-7-hydroxy-THIQ | 3-Methoxytyramine |
 | Lophocerine | 1-(2-Methylpropyl)-2-methyl-6-methoxy-7-hydroxy-THIQ | 3-Methoxytyramine |
 | Heliamine | 6,7-Dimethoxy-THIQ | 3,4-Dimethoxyphenethylamine |
 | Salsolidine | 1-Methyl-6,7-dimethoxy-THIQ | 3,4-Dimethoxyphenethylamine |
 | N-Methylheliamine | 2-Methyl-6,7-dimethoxy-THIQ | 3,4-Dimethoxyphenethylamine |
 | Lemaireocereine | 7,8-Dimethoxy-THIQ | 3,4-Dimethoxyphenethylamine |
 | Carnegine | 1,2-Dimethyl-6,7-dimethoxy-THIQ | 3,4-Dimethoxyphenethylamine |
 | Tepenine | 1,2-Dimethyl-7,8-dimethoxy-THIQ | 3,4-Dimethoxyphenethylamine |
 | Calycotomine | 1-(Hydroxymethyl)-6,7-dimethoxy-THIQ | 3,4-Dimethoxyphenethylamine |
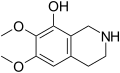 | Anhalamine | 6,7-Dimethoxy-8-hydroxy-THIQ | 3-Desmethylmescaline |
 | Isoanhalamine | 6-Hydroxy-7,8-dimethoxy-THIQ | 3-Desmethylmescaline |
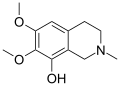 | Anhalidine | 2-Methyl-6,7-dimethoxy-8-hydroxy-THIQ | 3-Desmethylmescaline |
 | Isoanhalidine | 2-Methyl-6-hydroxy-7,8-dimethoxy-THIQ | 3-Desmethylmescaline |
 | Anhalonidine | 1-Methyl-6,7-dimethoxy-8-hydroxy-THIQ | 3-Desmethylmescaline |
 | Isoanhalonidine | 1-Methyl-6-hydroxy-7,8-dimethoxy-THIQ | 3-Desmethylmescaline |
 | Pellotine | 1,2-Dimethyl-6,7-dimethoxy-8-hydroxy-THIQ | 3-Desmethylmescaline |
 | Peyotine | 1,2,2-Trimethyl-6,7-dimethoxy-8-hydroxy-THIQ | 3-Desmethylmescaline |
 | Isopellotine | 1,2-Dimethyl-6-hydroxy-7,8-dimethoxy-THIQ | 3-Desmethylmescaline |
 | Gigantine | 1,2-Dimethyl-5-hydroxy-6,7-dimethoxy-THIQ | 2-Hydroxy-3,4-dimethoxyphenethylamine |
 | Deglucopterocereine | 1-(Hydroxymethyl)-2-methyl-5-hydroxy-6,7-dimethoxy-THIQ | 2-Hydroxy-3,4-dimethoxyphenethylamine |
 | Anhalinine | 6,7,8-Trimethoxy-THIQ | Mescaline |
 | N-Methylanhalinine | 2-Methyl-6,7,8-trimethoxy-THIQ | Mescaline |
 | O-Methylanhalonidine | 1-Methyl-6,7,8-trimethoxy-THIQ | Mescaline |
 | O-Methylpellotine | 1,2-Dimethyl-6,7,8-trimethoxy-THIQ | Mescaline |
 | Nortehaunine | 5,6,7-Trimethoxy-THIQ | Isomescaline |
 | Tehaunine | 2-Methyl-5,6,7-trimethoxy-THIQ | Isomescaline |
 | Hydrocotarnine | 2-Methyl-6,7-methylenedioxy-8-methoxy-THIQ | Lophophine |
 | Anhalonine | 1-Methyl-6-methoxy-7,8-methylenedioxy-THIQ | Lophophine |
 | Anhalotine | 2,2-Dimethyl-6,7-dimethoxy-8-hydroxy-THIQ | 3-Desmethylmescaline |
 | Lophophorine | 1,2-Dimethyl-6-methoxy-7,8-methylenedioxy-THIQ | Lophophine |
 | Lophotine | 1,2,2-Trimethyl-6-Methoxy-7,8-methylenedioxy-THIQ | Lophophine |
 | Peyophorine | 1-Methyl-2-ethyl-6-methoxy-7,8-methylenedioxy-THIQ | Lophophine |
 | Norweberine | 5,6,7,8-Tetramethoxy-THIQ | 2,3,4,5-Tetramethoxyphenethylamine |
 | Weberine | 2-Methyl-5,6,7,8-tetramethoxy-THIQ | 2,3,4,5-Tetramethoxyphenethylamine |
 | Pachycereine | 1-Methyl-5,6,7,8-tetramethoxy-THIQ | 2,3,4,5-Tetramethoxyphenethylamine |
 | Tehaunine N-oxide | 2-Methyl-2-oxo-5,6,7-trimethoxy-THIQ | Isomescaline |
Synthetic simple tetrahydroisoquinolines
[edit]| Structure | Name | Chemical Name | AMPH Counterpart |
|---|---|---|---|
 | Tetrahydroisoquinoline (THIQ; AMPH-CR)[10] | 1,2,3,4-Tetrahydroisoquinoline | Amphetamine |
| N-Methyl-THIQ (METH-CR)[10] | 2-Methyl-THIQ | Methamphetamine | |
| PMMA-CR[10] | 2-Methyl-7-methoxy-THIQ | para-Methoxymethamphetamine (PMMA) | |
 | DOM-CR[10] | 5,8-Dimethoxy-7-methyl-THIQ | 2,5-Dimethoxy-4-methylamphetamine (DOM) |
 | DOB-CR[10] | 5,8-Dimethoxy-7-bromo-THIQ | 2,5-Dimethoxy-4-bromoamphetamine (DOB) |
 | N-Methyl-DOM-CR[10] | 2,7-Dimethyl-5,8-dimethoxy-THIQ | Beatrice (N-methyl-DOM) |
| TDIQ (MDTHIQ, MDA-CR)[10] | 6,7-Methylenedioxy-THIQ | 3,4-Methylenedioxyamphetamine (MDA) | |
| Hydrohydrastinine (MDMTHIQ, MDMA-CR) | 2-Methyl-6,7-methylenedioxy-THIQ | 3,4-Methylenedioxymethamphetamine (MDMA) | |
 | Hydrastinine | 1-Hydroxy-2-methyl-6,7-methylenedioxy-THIQ | 3,4-Methylenedioxymethamphetamine (MDMA) |
See also
[edit]- The Simple Plant Isoquinolines (2002)
- Substituted phenethylamine
- Substituted β-carboline and harmala alkaloid
- Iboga-type alkaloid and ibogalog
Notes
[edit]- ^ With no amine substituents (e.g., methyl groups).
References
[edit]- ^ a b c d e Shulgin, Alexander Theodore; Perry, Wendy E. (2002). The Simple Plant Isoquinolines. Transform Press. ISBN 978-0-9630096-2-3. OCLC 51006569. OL 8521520M.
- ^ a b c d e f Keeper Trout & friends (2013). Trout's Notes on The Cactus Alkaloids Nomenclature, Physical properties, Pharmacology & Occurrences (Sacred Cacti Fourth Edition, Part C: Cactus Chemistry: Section 1) (PDF). Mydriatic Productions/Better Days Publishing.
- ^ Lundström, Jan (1983). "Chapter 6 Simple Isoquinoline Alkaloids". The Alkaloids: Chemistry and Pharmacology. Vol. 21. Elsevier. pp. 255–327. doi:10.1016/s0099-9598(08)60052-8. ISBN 978-0-12-469521-4.
TABLE 1: SIMPLE ISOQUINOLINE ALKALOIDS [...] TABLE II SIMPLE ISOQUINOLINE ALKALOIDS IN THE FAMILY OF CACTACEAE [...]
- ^ a b c Cassels, Bruce K. (2019). "Alkaloids of the Cactaceae — The Classics". Natural Product Communications. 14 (1). doi:10.1177/1934578X1901400123. ISSN 1934-578X.
- ^ Rommelspacher H, Susilo R (1985). "Tetrahydroisoquinolines and beta-carbolines: putative natural substances in plants and mammals". Prog Drug Res. 29: 415–459. doi:10.1007/978-3-0348-9315-2_10. ISBN 978-3-0348-9992-5. PMID 3911263.
- ^ a b Poulie CB, Chan CB, Parka A, Lettorp M, Vos J, Raaschou A, Pottie E, Bundgaard MS, Sørensen LM, Cecchi CR, Märcher-Rørsted E, Bach A, Herth MM, Decker A, Jensen AA, Elfving B, Kretschmann AC, Stove CP, Kohlmeier KA, Cornett C, Janfelt C, Kornum BR, Kristensen JL (October 2023). "In Vitro and In Vivo Evaluation of Pellotine: A Hypnotic Lophophora Alkaloid". ACS Pharmacol Transl Sci. 6 (10): 1492–1507. doi:10.1021/acsptsci.3c00142. PMC 10580395. PMID 37854625.
- ^ a b Chan CB, Pottie E, Simon IA, Rossebø AG, Herth MM, Harpsøe K, Kristensen JL, Stove CP, Poulie CB (February 2025). "Synthesis, Pharmacological Characterization, and Binding Mode Analysis of 8-Hydroxy-Tetrahydroisoquinolines as 5-HT7 Receptor Inverse Agonists". ACS Chem Neurosci. 16 (3): 439–451. doi:10.1021/acschemneuro.4c00667. PMID 39836645.
- ^ a b Malmusi, L., Dukat, M., Young, R., Teitler, M., Darmani, N. A., Ahmad, B., ... & Glennon, R. A. (1996). 1, 2, 3, 4-Tetrahydroisoquinoline analogs of phenylalkylamine stimulants and hallucinogens. Medicinal Chemistry Research, 6(6), 400–411. https://scholar.google.com/scholar?cluster=16646102221398485716 "Conformationally constrained, 1,2,3,4-tetrahydroisoquinoline (TIQ) analogs of central stimulant (e.g. amphetamine) and hallucinogenic (e.g. DOM) phenylalkylamines were prepared and evaluated to determine the contribution to activity of this conformational restriction. The amphetamine-related TIQs failed to produce locomotor stimulation in mice and did not produce amphetamine-appropriate responding in tests of stimulus generalization in (+)amphetamine-trained rats. Hallucinogen-related TIQs lacked appreciable affinity for 5-HT2A serotonin receptors and did not produce DOM-like effects in tests of stimulus generalization in DOM-trained rats. It is concluded that the phenylalkylamine conformation represented by the TIQs is not a major contributor to these actions."
- ^ a b Malmusi, L., Dukat, M., Young, R., Teitler, M., Darmani, N. A., Ahmad, B., ... & Glennon, R. A. (1996). 1,2,3,4-Tetrahydroisoquinoline and related analogs of the phenylalkylamine designer drug MDMA. Medicinal Chemistry Research, 6(6), 412–426. https://scholar.google.com/scholar?cluster=15073179555289853539 "1,2,3,4-Tetrahydroisoquinoline (TIQ) analogs of 1-(3,4-methylenedioxyphenyl)-2-aminopropane (MDA) and its N-methyl derivative, MDMA, similar in structure to a TIQ metabolite of MDA, were prepared and examined (a) in tests of central stimulant activity in mice, (b) for their ability to bind at human 5-HT2A receptors, and (c) in tests of stimulus generalization in rats trained to discriminate MDMA from vehicle. In general, the TIQ analogs failed to display appreciable activity in any assay system. Conversely, certain 2-aminotetralin and 2-aminoindan analogs were active in the stimulus generalization studies. It is concluded that TIQ-like conformations do not account for the actions typically associated with MDA- and MDMA-related agents."
- ^ a b c d e f g h i j Glennon RA, Young R, Rangisetty JB (May 2002). "Further characterization of the stimulus properties of 5,6,7,8-tetrahydro-1,3-dioxolo[4,5-g]isoquinoline". Pharmacol Biochem Behav. 72 (1–2): 379–387. doi:10.1016/s0091-3057(01)00768-7. PMID 11900809.
Nonetheless, it appears that conformational restriction of phenylalkylamine hallucinogens, stimulants, and designer drugs into a tetrahydroisoquinoline structure usually abolishes their respective actions (Malmusi et al., 1996b; Young et al., 1999a,b). [...] Table 6 Summary of stimulus generalization results of agents used in the present investigation [...]
- ^ a b Young R, Glennon RA (2002). "The stimulus effect of 5,6,7,8-tetrahydro-1,3-dioxolo[4,5-g]isoquinoline is similar to that of cocaine but different from that of amphetamine". Pharmacol Biochem Behav. 71 (1–2): 205–213. doi:10.1016/s0091-3057(01)00666-9. PMID 11812524.
- ^ a b c Young R (2007). "TDIQ (5,6,7,8-tetrahydro-1,3-dioxolo [4,5-g]isoquinoline): discovery, pharmacological effects, and therapeutic potential". CNS Drug Rev. 13 (4): 405–422. doi:10.1111/j.1527-3458.2007.00022.x. PMC 6494129. PMID 18078426.
External links
[edit]- Structural Tables: Isoquinolines - Trout's Notes
- Isoquinolines: Structural Analogues of the Mysterious Third Family - Nikita Obidin