Ductal carcinoma in situ
 From Wikipedia the free encyclopedia
From Wikipedia the free encyclopedia
This article needs to be updated. The reason given is: almost every source in this article is around fifteen years old. (June 2022) |
| Breast cancer in situ | |
|---|---|
| Other names | Intraductal carcinoma |
 | |
| Ducts of the mammary gland, the location of ductal carcinoma | |
| Specialty | Oncology |

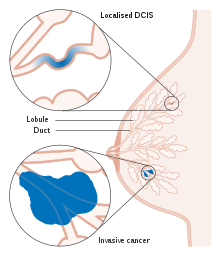
Ductal carcinoma in situ (DCIS), also known as intraductal carcinoma, is a pre-cancerous or non-invasive cancerous lesion of the breast.[1][2] DCIS is classified as Stage 0.[3] It rarely produces symptoms or a breast lump that can be felt, typically being detected through screening mammography.[4][5] It has been diagnosed in a significant percentage of men (see male breast cancer).[6]
In DCIS, abnormal cells are found in the lining of one or more milk ducts in the breast. In situ means "in place" and refers to the fact that the abnormal cells have not moved out of the mammary duct and into any of the surrounding tissues in the breast ("pre-cancerous" indicates that it has not yet become an invasive cancer). In some cases, DCIS may become invasive and spread to other tissues, but there is no way of determining which lesions will remain stable without treatment, and which will go on to become invasive.[7] DCIS encompasses a wide spectrum of diseases ranging from low-grade lesions that are not life-threatening to high-grade (i.e. potentially highly aggressive) lesions.
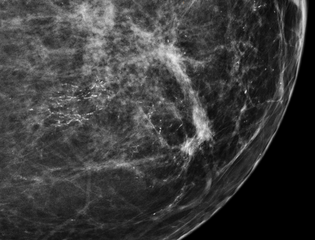
DCIS has been classified according to the architectural pattern of the cells (solid, cribriform, papillary, and micropapillary), tumor grade (high, intermediate, and low grade), or the presence or absence of comedo histology;[8] or, in the case of the apocrine cell-based in situ carcinoma, apocrine ductal carcinoma in situ, it may be classified according to the cell type forming the lesion.[9] DCIS can be detected on mammograms by examining tiny specks of calcium known as microcalcifications. Since suspicious groups of microcalcifications can appear even in the absence of DCIS, a biopsy may be necessary for diagnosis.
About 20–30% of those who do not receive treatment develop breast cancer.[10][11] DCIS is the most common type of pre-cancer in women. There is some disagreement on its status as cancer; some bodies include DCIS when calculating breast cancer statistics, while others do not.[12][13]
Terminology[edit]
Ductal carcinoma in situ (DCIS) literally means groups of "cancerous" epithelial cells which remain in their normal location (in situ) within the ducts and lobules of the mammary gland.[14] Clinically, DCIS is considered to be a premalignant (i.e. potentially malignant) condition,[15] because the biologically abnormal cells have not yet crossed the basement membrane to invade the surrounding tissue.[14][16] When multiple lesions (known as "foci" of DCIS) are present in different quadrants of the breast, this is referred to as "multicentric" disease.[8]
For statistical purposes, DCIS is sometimes counted as a "cancer", but this is not always the case.[13][17] When classified as a cancer, it is referred to as a "non-invasive" or "pre-invasive" form.[14][18] It is described by the National Cancer Institute as a "noninvasive condition".[13]
Signs and symptoms[edit]

Most of the women who develop DCIS do not experience any symptoms. The majority of cases (80-85%) are detected through screening mammography. The first signs and symptoms may appear if the cancer advances. Because of the lack of early symptoms, DCIS is most often detected at screening mammography.
In a few cases, DCIS may cause:
- A lump or thickening in or near the breast or under the arm
- A change in the size or shape of the breast
- Nipple discharge or nipple tenderness; the nipple may also be inverted, or pulled back into the breast
- Ridges or pitting of the breast; the skin may look like the skin of an orange
- A change in the way the skin of the breast, areola, or nipple looks or feels[19] such as warmth, swelling, redness or scaliness.[20]
Causes[edit]
The specific causes of DCIS are still unknown. The risk factors for developing this condition are similar to those for invasive breast cancer.[21]
Some women are however more prone than others to developing DCIS. Women considered at higher risks are those who have a family history of breast cancer, those who have had their periods at an early age or who have had a late menopause. Also, women who have never had children or had them late in life are also more likely to get this condition.
Long-term use of estrogen-progestin hormone replacement therapy (HRT) for more than five years after menopause, genetic mutations (BRCA1 or BRCA2 genes), atypical hyperplasia, as well as radiation exposure or exposure to certain chemicals may also contribute in the development of the condition.[22] Nonetheless, the risk of developing noninvasive cancer increases with age and it is higher in women older than 45 years.
Diagnosis[edit]
80% of cases in the United States are detected by mammography screening.[23] More definitive diagnosis is made by breast biopsy for histopathology.
- Mammogram microcalcifications in ductal carcinoma in situ
- Histopathology of dystrophic microcalcifications in DCIS, H&E stain.
- Histopathologic architectural patterns of DCIS.[24]
- DCIS with microinvasion, defined as focus of invasive cancer measuring up to 1.0 mm in size.[26]
- Immunohistochemistry for calponin in ductal carcinoma in situ, highlighting myoepithelial cells around all tumor cells, thereby ruling out invasive ductal carcinoma.
- Ductal carcinoma in situ with comedo necrosis spanning 30% of its diameter, which is generally regarded as the minimal size to classify it as comedo.[27]
Treatment[edit]
There are different opinions on the best treatment of DCIS.[28] Surgical removal, with or without additional radiation therapy or tamoxifen, is the recommended treatment for DCIS by the National Cancer Institute.[29] Surgery may be either a breast-conserving lumpectomy or a mastectomy (complete or partial removal of the affected breast).[30] If a lumpectomy is used it is often combined with radiation therapy.[13] Tamoxifen may be used as hormonal therapy if the cells show estrogen receptor positivity.[13] Research shows that survival is the same with lumpectomy as it is with mastectomy, whether or not a woman has radiation after lumpectomy.[31] Chemotherapy is not needed for DCIS since the disease is noninvasive.[32]
While surgery reduces the risk of subsequent cancer, many people never develop cancer even without treatment and the associated side effects.[30] There is no evidence comparing surgery with watchful waiting and some feel watchful waiting may be a reasonable option in certain cases.[30]
Radiation therapy[edit]
Use of radiation therapy after lumpectomy provides equivalent survival rates to mastectomy, although there is a slightly higher risk of recurrent disease in the same breast in the form of further DCIS or invasive breast cancer. Systematic reviews (including a Cochrane review) indicate that the addition of radiation therapy to lumpectomy reduces recurrence of DCIS or later onset of invasive breast cancer in comparison with breast-conserving surgery alone, without affecting mortality.[33][34][35] The Cochrane review did not find any evidence that the radiation therapy had any long-term toxic effects.[33] While the authors caution that longer follow-up will be required before a definitive conclusion can be reached regarding long-term toxicity, they point out that ongoing technical improvements should further restrict radiation exposure in healthy tissues.[33] They do recommend that comprehensive information on potential side effects is given to women who receive this treatment.[33] The addition of radiation therapy to lumpectomy appears to reduce the risk of local recurrence to approximately 12%, of which approximately half will be DCIS and half will be invasive breast cancer; the risk of recurrence is 1% for women undergoing mastectomy.[36]
Mastectomy[edit]
There is no evidence that mastectomy decreases the risk of death over a lumpectomy.[37] Mastectomy, however, may decrease the rate of the DCIS or invasive cancer occurring in the same location.[7][37]
Mastectomies remain a common recommendation in those with persistent microscopic involvement of margins after local excision or with a diagnosis of DCIS and evidence of suspicious, diffuse microcalcifications.[38]
Sentinel node biopsy[edit]
Some institutions that have encountered high rates of recurrent invasive cancers after mastectomy for DCIS have endorsed routine sentinel node biopsy (SNB).[39] However, research indicates that sentinel node biopsy has risks that outweigh the benefits for most women with DCIS.[40] SNB should be considered with tissue diagnosis of high-risk DCIS (grade III with palpable mass or larger size on imaging) as well as in people undergoing mastectomy after a core or excisional biopsy diagnosis of DCIS.[41][42]
Prognosis[edit]
With treatment, the prognosis is excellent, with greater than 97% long-term survival. If untreated, DCIS progresses to invasive cancer in roughly one-third of cases, usually in the same breast and quadrant as the earlier DCIS.[43] About 2% of women who are diagnosed with this condition and treated died within 10 years.[44] Biomarkers can identify which women who were initially diagnosed with DCIS are at high or low risk of subsequent invasive cancer.[45][46]
Epidemiology[edit]

DCIS is often detected with mammographies but can rarely be felt. With the increasing use of screening mammography, noninvasive cancers are more frequently diagnosed and now constitute 15% to 20% of all breast cancers.[38]
Cases of DCIS have increased five-fold between 1983 and 2003 in the United States due to the introduction of screening mammography.[44] In 2009 about 62,000 cases were diagnosed.[44]
References[edit]
- ^ Sinn, HP; Kreipe, H (May 2013). "A Brief Overview of the WHO Classification of Breast Tumors, 4th Edition, Focusing on Issues and Updates from the 3rd Edition". Breast Care. 8 (2): 149–154. doi:10.1159/000350774. PMC 3683948. PMID 24415964.
- ^ Hindle, William H. (1999). Breast Care: A Clinical Guidebook for Women’s Primary Health Care Providers. New York: Springer. p. 129. ISBN 978-0-387-98348-6.
- ^ DePolo, Jamie (13 October 2023). "Breast Cancer Stages: Stage 0 breast cancer". Breastcancer.org.
- ^ Welch HG, Woloshin S, Schwartz LM (February 2008). "The sea of uncertainty surrounding ductal carcinoma in situ--the price of screening mammography". J. Natl. Cancer Inst. 100 (4): 228–9. doi:10.1093/jnci/djn013. PMID 18270336.
- ^ Morris, Elizabeth A.; Liberman, Laura, eds. (2005). Breast MRI: Diagnosis and Intervention. New York: Springer. p. 164. ISBN 978-0-387-21997-4.
- ^ Nofal MN, Yousef AJ (December 2019). "The diagnosis of male breast cancer". The Netherlands Journal of Medicine. 77 (10): 356–359. PMID 31880271.
- ^ a b Mannu, GS; Wang, Z; Broggio, J; Charman, J; Cheung, S; Kearins, O; Dodwell, D; Darby, SC (27 May 2020). "Invasive breast cancer and breast cancer mortality after ductal carcinoma in situ in women attending for breast screening in England, 1988-2014: population based observational cohort study". BMJ (Clinical Research Ed.). 369: m1570. doi:10.1136/bmj.m1570. PMC 7251423. PMID 32461218.
- ^ a b Virnig BA, Shamliyan T, Tuttle TM, Kane RL, Wilt TJ (September 2009). "Diagnosis and management of ductal carcinoma in situ (DCIS)". Evidence Report/Technology Assessment. AHRQ Publication No.09-E018. (185): 1–549. PMC 4781639. PMID 20629475.
- ^ Quinn CM, D'Arcy C, Wells C (January 2022). "Apocrine lesions of the breast". Virchows Archiv. 480 (1): 177–189. doi:10.1007/s00428-021-03185-4. PMC 8983539. PMID 34537861.
- ^ Rubin, Raphael; Strayer, David S., eds. (2008). Rubin's Pathology: Clinicopathologic Foundations of Medicine (5th ed.). Philadelphia: Lippincott Williams and Wilkins. p. 848. ISBN 978-0-7817-9516-6.
- ^ Early Breast Cancer Trialists' Collaborative Group (EBCTCG); Correa, C.; McGale, P.; Taylor, C.; Wang, Y.; Clarke, M.; Davies, C.; Peto, R.; Bijker, N. (2010). "Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast". Journal of the National Cancer Institute. Monographs. 2010 (41): 162–177. doi:10.1093/jncimonographs/lgq039. ISSN 1745-6614. PMC 5161078. PMID 20956824.
- ^ "Breast Cancer Treatment (PDQ®)". NCI. 11 April 2014. Retrieved 19 June 2014.
- ^ a b c d e "Breast Cancer Treatment (PDQ®)". NCI. January 1980. Retrieved 19 June 2014.
- ^ a b c Allred DC (2010). "Ductal carcinoma in situ: terminology, classification, and natural history". Journal of the National Cancer Institute. Monographs. 2010 (41): 134–8. doi:10.1093/jncimonographs/lgq035. PMC 5161057. PMID 20956817.
- ^ Hui, David; Leung, Alexander A.; Padwal, Raj, eds. (2011). Approach to Internal Medicine: A Resource Book for Clinical Practice (3rd ed.). New York: Springer. p. 198. ISBN 978-1-4419-6505-9.
- ^ Tjandra, Joe J.; Collins, John P. (2006). "Breast surgery". In Tjandra; et al. (eds.). Textbook of surgery (3rd ed.). Malden, Mass.: Blackwell Pub. p. 282. ISBN 9780470757796.
- ^ Chang, Alfred E.; Ganz, Patricia A.; Hayes, Daniel F.; et al., eds. (2007). Oncology: An Evidence-Based Approach. Springer. p. 162. ISBN 978-0-387-31056-5.
- ^ Saclarides, Theodore J.; Myers, Jonathan A.; Millikan, Keith W., eds. (2008). Common Surgical Diseases: An Algorithmic Approach to Problem Solving (2nd revised ed.). New York: Springer. ISBN 978-0-387-75246-4.
- ^ "Breast Cancer". Retrieved 28 June 2010.
- ^ "Signs and Symptoms". Retrieved 28 June 2010.
- ^ "After the mammogram". Archived from the original on 7 April 2010. Retrieved 28 June 2010.
- ^ "Intraductal Carcinoma of the Breast". Archived from the original on 11 June 2010. Retrieved 28 June 2010.
- ^ "Ductal Carcinoma In Situ". cancer.gov. 9 January 2015. Retrieved 5 March 2015.
- ^ Top and bottom left images by Mikael Häggström, MD. Bottom right image from:
Kulka, J.; Madaras, L.; Floris, G.; Lax, S.F. (2022). "Papillary lesions of the breast". Virchows Arch. 480 (1): 65–84. doi:10.1007/s00428-021-03182-7. PMC 8983543. PMID 34734332.
- "This article is licensed under a Creative Commons Attribution 4.0 International License" - ^ Image by Mikael Häggström, MD. References for features:
- "Ductal Carcinoma in Situ of the Breast". Stanford Medical School. 27 August 2020. Archived from the original on 30 March 2023. Retrieved 29 October 2023.
- Hayward, M.K.; Louise Jones, J.; Hall, A.; King, L.; Ironside, A.J.; Nelson, A.C.; et al. (2020). "Derivation of a nuclear heterogeneity image index to grade DCIS". Comput Struct Biotechnol J. 18: 4063–4070. doi:10.1016/j.csbj.2020.11.040. PMC 7744935. PMID 33363702. - ^ Image annotation by Mikael Häggström, MD, using source image from:
Moatasim A, Mamoon N (2022). "Primary Breast Mucinous Cystadenocarcinoma and Review of Literature". Cureus. 14 (3): e23098. doi:10.7759/cureus.23098. PMC 8997314. PMID 35464581.
- "This is an open access article distributed under the terms of the Creative Commons Attribution License CC BY 4.0."
Source for microinvasion: "Protocol for the Examination of Resection Specimens from Patients with Ductal Carcinoma In Situ (DCIS) of the Breast, Version: 4.4.0.0. Protocol Posting Date: June 2021" (PDF). College of American Pathologists. - ^ Image by Mikael Häggström, MD.
- Reference for 30% being the most common definition of comedo necrosis by size:
- Harrison, B.T.; Hwang, E.S.; Partridge, A.H.; Thompson, A.M.; Schnitt, S.J. (2019). "Variability in diagnostic threshold for comedo necrosis among breast pathologists: implications for patient eligibility for active surveillance trials of ductal carcinoma in situ". Mod Pathol. 32 (9): 1257–1262. doi:10.1038/s41379-019-0262-4. PMID 30980039. - ^ Mannu, Gurdeep S.; Bettencourt-Silva, Joao H.; Ahmed, Farid; Cunnick, Giles (2015). "A Nationwide Cross-Sectional Survey of UK Breast Surgeons' Views on the Management of Ductal Carcinoma In Situ". International Journal of Breast Cancer. 2015: 104231. doi:10.1155/2015/104231. PMC 4677188. PMID 26697227.
- ^ "Ductal Carcinoma In Situ: Treatment Options for Patients With DCIS". National Cancer Institute at NIH. National Institutes of Health. 11 July 2014.
- ^ a b c "Treatment of ductal carcinoma in situ: an uncertain harm-benefit balance". Prescrire Int. 22 (144): 298–303. December 2013. PMID 24600734.
- ^ J, Cuzick; I, Sestak; Se, Pinder; Io, Ellis; S, Forsyth; Nj, Bundred; Jf, Forbes; H, Bishop; Is, Fentiman (January 2011). "Effect of Tamoxifen and Radiotherapy in Women With Locally Excised Ductal Carcinoma in Situ: Long-Term Results From the UK/ANZ DCIS Trial". The Lancet. Oncology. 12 (1): 21–9. doi:10.1016/S1470-2045(10)70266-7. PMC 3018565. PMID 21145284.
- ^ Ductal Carcinoma in Situ (DCIS) Archived 24 April 2015 at the Wayback Machine, Johns Hopkins Medicine
- ^ a b c d Goodwin A, Parker S, Ghersi D, Wilcken N (2013). "Post-operative radiotherapy for ductal carcinoma in situ of the breast". The Cochrane Database of Systematic Reviews. 11 (11): CD000563. doi:10.1002/14651858.CD000563.pub7. PMID 24259251.
- ^ Virnig, BA; Tuttle, TM; Shamliyan, T; Kane, RL (2010). "Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes". Journal of the National Cancer Institute. 102 (3): 170–8. doi:10.1093/jnci/djp482. PMID 20071685.
- ^ Correa, C.; McGale, P.; Taylor, C.; Wang, Y.; Clarke, M.; Davies, C.; Peto, R.; Bijker, N.; Solin, L.; Darby, S.; Darby, S (2010). "Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast". Journal of the National Cancer Institute. Monographs. 2010 (41): 162–177. doi:10.1093/jncimonographs/lgq039. ISSN 1745-6614. PMC 5161078. PMID 20956824.
- ^ "NIH DCIS Consensus Conference Statement". National Institutes of Health. September 2009. Archived from the original on 13 August 2011. Retrieved 19 June 2014.
- ^ a b Virnig, BA; Shamliyan, T; Tuttle, TM; Kane, RL; Wilt, TJ (September 2009). "Diagnosis and management of ductal carcinoma in situ (DCIS)". Evidence Report/Technology Assessment (185): 4. PMC 4781639. PMID 20629475.
They found women undergoing mastectomy were less likely than women undergoing lumpectomy plus radiation to experience local DCIS or invasive recurrence. Women undergoing BCS alone were also more likely to experience a local recurrence than women treated with mastectomy. We found no study showing a mortality reduction associated with mastectomy over breast conserving surgery with or without radiation
- ^ a b "Intraductal carcinoma". Archived from the original on 10 April 2016. Retrieved 28 June 2010.
- ^ Tan JC, McCready DR, Easson AM, Leong WL (February 2007). "Role of sentinel lymph node biopsy in ductal carcinoma-in-situ treated by mastectomy". Annals of Surgical Oncology. 14 (2): 638–45. doi:10.1245/s10434-006-9211-9. PMID 17103256. S2CID 1924867.
- ^ Hung, Peiyin; Wang, Shi-Yi; Killelea, Brigid K.; Mougalian, Sarah S.; Evans, Suzanne B.; Sedghi, Tannaz; Gross, Cary P. (1 December 2019). "Long-Term Outcomes of Sentinel Lymph Node Biopsy for Ductal Carcinoma in Situ". JNCI Cancer Spectrum. 3 (4): pkz052. doi:10.1093/jncics/pkz052. PMC 7049982. PMID 32337481.
- ^ Mannu, GS; Groen, EJ; Wang, Z; Schaapveld, M; Lips, EH; Chung, M; Joore, I; van Leeuwen, FE; Teertstra, HJ; Winter-Warnars, GAO; Darby, SC; Wesseling, J (November 2019). "Reliability of preoperative breast biopsies showing ductal carcinoma in situ and implications for non-operative treatment: a cohort study". Breast Cancer Research and Treatment. 178 (2): 409–418. doi:10.1007/s10549-019-05362-1. PMC 6797705. PMID 31388937.
- ^ van Deurzen CH, Hobbelink MG, van Hillegersberg R, van Diest PJ (April 2007). "Is there an indication for sentinel node biopsy in patients with ductal carcinoma in situ of the breast? A review". European Journal of Cancer. 43 (6): 993–1001. doi:10.1016/j.ejca.2007.01.010. PMID 17300928.
- ^ Basic Pathology, Robbins (2018). Breast. Copyright © 2018 by Elsevier Inc. p. 743. ISBN 978-0-323-35317-5.
- ^ a b c Kerlikowske, K (2010). "Epidemiology of ductal carcinoma in situ". Journal of the National Cancer Institute. Monographs. 2010 (41): 139–41. doi:10.1093/jncimonographs/lgq027. PMC 5161058. PMID 20956818.
- ^ Kerlikowske, K.; Molinaro, A. M.; Gauthier, M. L.; Berman, H. K.; Waldman, F.; Bennington, J.; Sanchez, H.; Jimenez, C.; Stewart, K.; et al. (2010). "Biomarker Expression and Risk of Subsequent Tumors After Initial Ductal Carcinoma in Situ Diagnosis". JNCI Journal of the National Cancer Institute. 102 (9): 627–637. doi:10.1093/jnci/djq101. PMC 2864293. PMID 20427430.
- ^ Witkiewicz AK, Dasgupta A, Nguyen KH, et al. (June 2009). "Stromal caveolin-1 levels predict early DCIS progression to invasive breast cancer". Cancer Biology & Therapy. 8 (11): 1071–1079. doi:10.4161/cbt.8.11.8874. PMID 19502809.

![Histopathologic architectural patterns of DCIS.[24]](http://upload.wikimedia.org/wikipedia/commons/thumb/5/58/Histopathologic_architectural_patterns_of_DCIS.png/288px-Histopathologic_architectural_patterns_of_DCIS.png)
![Histopathology of high-grade DCIS. H&E stain. RBC = red blood cell.[25]](http://upload.wikimedia.org/wikipedia/commons/thumb/3/3f/Histopathology_of_high-grade_DCIS.png/202px-Histopathology_of_high-grade_DCIS.png)
![DCIS with microinvasion, defined as focus of invasive cancer measuring up to 1.0 mm in size.[26]](http://upload.wikimedia.org/wikipedia/commons/thumb/d/d9/Histopathology_of_microinvasive_ductal_carcinoma_in_situ.png/289px-Histopathology_of_microinvasive_ductal_carcinoma_in_situ.png)

![Ductal carcinoma in situ with comedo necrosis spanning 30% of its diameter, which is generally regarded as the minimal size to classify it as comedo.[27]](http://upload.wikimedia.org/wikipedia/commons/thumb/4/41/Histopathology_of_ductal_carcinoma_in_situ_with_comedo_necrosis.jpg/321px-Histopathology_of_ductal_carcinoma_in_situ_with_comedo_necrosis.jpg)